A Great 13C NMR Spectrum Even When Your Sample is Dilute
A great source of satisfaction in the chemistry lab is seeing a beautiful nuclear magnetic resonance (NMR) spectrum that gives you all the information you want. A beautiful 13C NMR spectrum means that the signal to noise ratio is large enough to see all of the resonances (visible as peaks), the peaks are tall and sharp and the baseline is flat.
Have you ever had trouble producing such spectra? It can be frustrating when your 13C NMR spectrum is missing peaks or taking a very long time. So what’s the problem? It could be that the molecule you are studying is in short supply and that you only have tens of milligrams to work with. So when you dissolve your molecules in deuterated solvent, the NMR sample is dilute.
It can be challenging to obtain a 13C NMR spectrum if the concentration of your solute in the NMR sample is low. This is a common problem in low-field NMR spectroscopy, and we want to help you fix it.
Remember the basics of sample preparation for NMR analysis
Use clean NMR tubes, a clean pasteur pipette, uncontaminated and dry deuterated solvents, purified organic compounds and a reference standard like tetramethylsilane (TMS). You might be able to get away with less than perfect technique when your sample is concentrated, but not so with diluted samples.
In addition, we recommend three other steps that should help you produce better quality 13C NMR spectra. These include increasing the concentration of your sample, using a shorter pulse width and using the Block Averaging with Peak Registration (BAPR) acquisition program.
Increase the NMR sample concentration as much as possible
How can you increase the concentration of solute in the NMR solvent? It helps to know these three scenarios that can lead to a low sample concentration:
- Using too much solvent for the mass of sample.
- Having a very small mass of material to work with.
- Using a sample that isn’t very soluble in your NMR solvent.
Here are three approaches that may help you to address these scenarios:
1. Know the necessary sample depth for your NMR probe and eliminate unneccessary solvent use.
The maximum volume of solvent you need for standard 5 mm NMR tubes is about 500 uL (0.5 mL). The NMR probe contains a radio frequency (RF) coil that excites the carbon-13 nuclei in your sample. The same RF coil also receives the signal produced as the carbon-13 nuclei relax. This coil is small and is positioned so that you only need 4 cm of solution in the NMR tube. Depending on the diameter of the NMR tube, this is about 400 ul for a medium-wall tube and 450-500 ul for a thin-wall tube.
Using more solvent than necessary will inevitably lead to a more dilute sample and require more scans in your NMR experiment. On the other hand, since the signal to noise ratio (S/N) increases as the square root of the number of additional scans, reducing the solvent by half reduces the number of extra scans (i.e. extra time) by a factor of four.
Please note that the active part of the RF coil length is actually less than 4 cm. This means that some of the solute molecules are above the coil and some are below. Consequently, carbon-13 nuclei in those molecules are not excited by the RF pulses. If they are not excited, they do not relax and therefore do not produce NMR signals. Only those carbon-13 nuclei within the active coil length are excited by each RF pulse. Consequently some of the carbon-13 nuclei in your precious molecules do not produce signals.
You might wonder, “Why can’t I just fill the NMR tube to a depth equal to the length of the active part of the RF coil? Then I could position the bottom of the tube at the bottom of the active part of the RF coil.” However the rounded bottom of the NMR tube interferes with the homogeneity of the magnetic field within the RF coil. This could reduce the sensitivity and impact the quality of your carbon NMR spectra.
Fortunately there is a better solution – special NMR tubes.
2. Use a special NMR tube to maximize signal
When you only have milligrams of compound to make a 13C NMR sample, you should take care to excite every single carbon-13 nuclei in your sample with each pulse from the RF coil of the probe. This will ensure maximum signal from your precious material and minimize the number of scans and time to perform the experiment.
But we just said that the liquid sample needs to occupy 4 cm in the NMR tube. How can you preserve this height while reducing the amount of solvent? The key is to use an NMR tube fitted with Doty susceptibility plugs as shown in Figure 1. Susceptibility plugs allow you to constrain the limited mass of compound to within the active part of the RF coil and therefore produce the maximum signal.
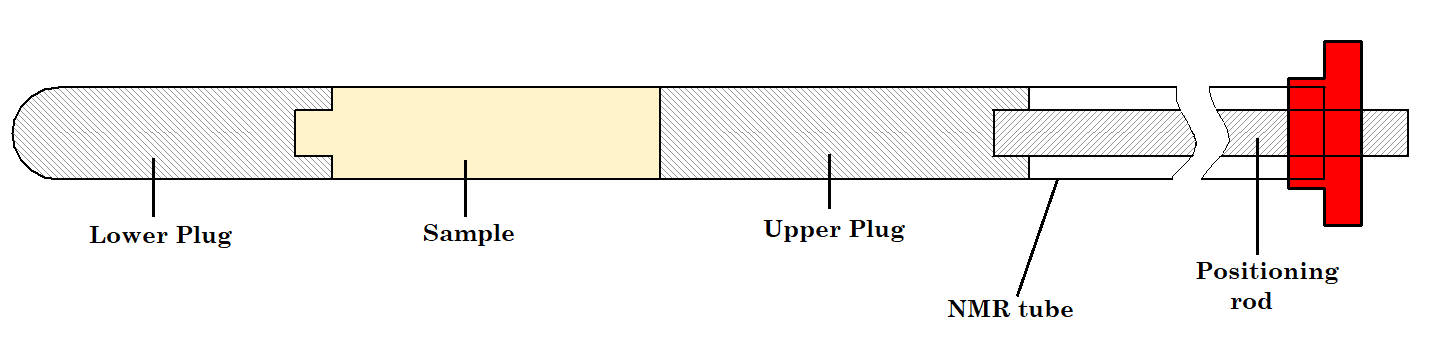
Figure 1. Doty susceptibility-matched sample plugs.
3. Experiment with multiple solvents to maximize the concentration of your NMR sample.
Often, the solubility of a material can present a problem as well. There may not be much you can do for this scenario. However, sometimes a mixture of different deuterated solvents can increase the amount of material that dissolves. In turn, your NMR sample will be more concentrated. Use your understanding of solubility, polarity and aromaticity to find the best solvent system for your sample.
Shorten the Pulse Width
Even in concentrated samples, the ability to see quaternary carbons or carbons without directly attached protons in the 13C NMR spectrum can be difficult. So, in the case of a dilute sample the use of a shorter pulse width is all the more desirable. While protonated carbons will end up a bit smaller, the carbons without protons will see a tremendous increase in signal.
Pulse Width (degrees) |
Transverse Magnetization |
Axial Magnetization |
| 30 | 50.0 | 86.6 |
| 60 | 86.6 | 50.0 |
| 90 | 100.0 | 0.0 |
Chart 1
Chart 1 shows the amount of magnetization transferred into the XY plane after a pulse. Before the pulse, the net magnetization is aligned along the Z-axis (axial magnetization). Pulsing 90 degrees transfers the net magnetization into the XY-plane (transverse magnetization). As the pulse width is shortened, less magnetization is transferred into the transverse plane, thereby reducing the signal. Up to this point we have done everything we can to increase signal from the carbon-13 nuclei. So why are we now saying that reducing the signal is a good approach? It is because shortened pulse widths result in significantly shorter relaxation times. That means that we have to wait less time between RF pulses; over the course of a long experiment with many many scans, more signal is actually produced in a shorter time frame.
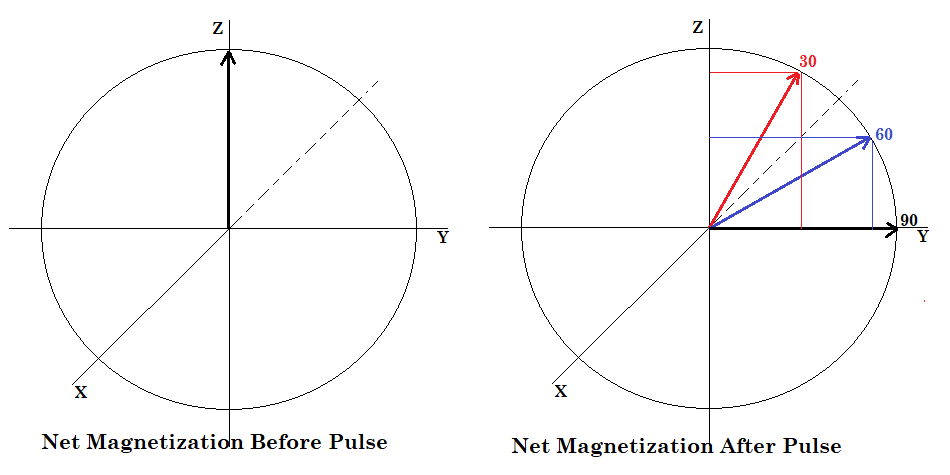
Figure 2.
The results speak for themselves. The carbonyl peak (165 ppm) is almost invisible in the 90-degree spectrum (Figure 3, top) while it is greatly enhanced in both the 60-degree (Figure 3, middle) and 30-degree (Figure 3, bottom) spectra.
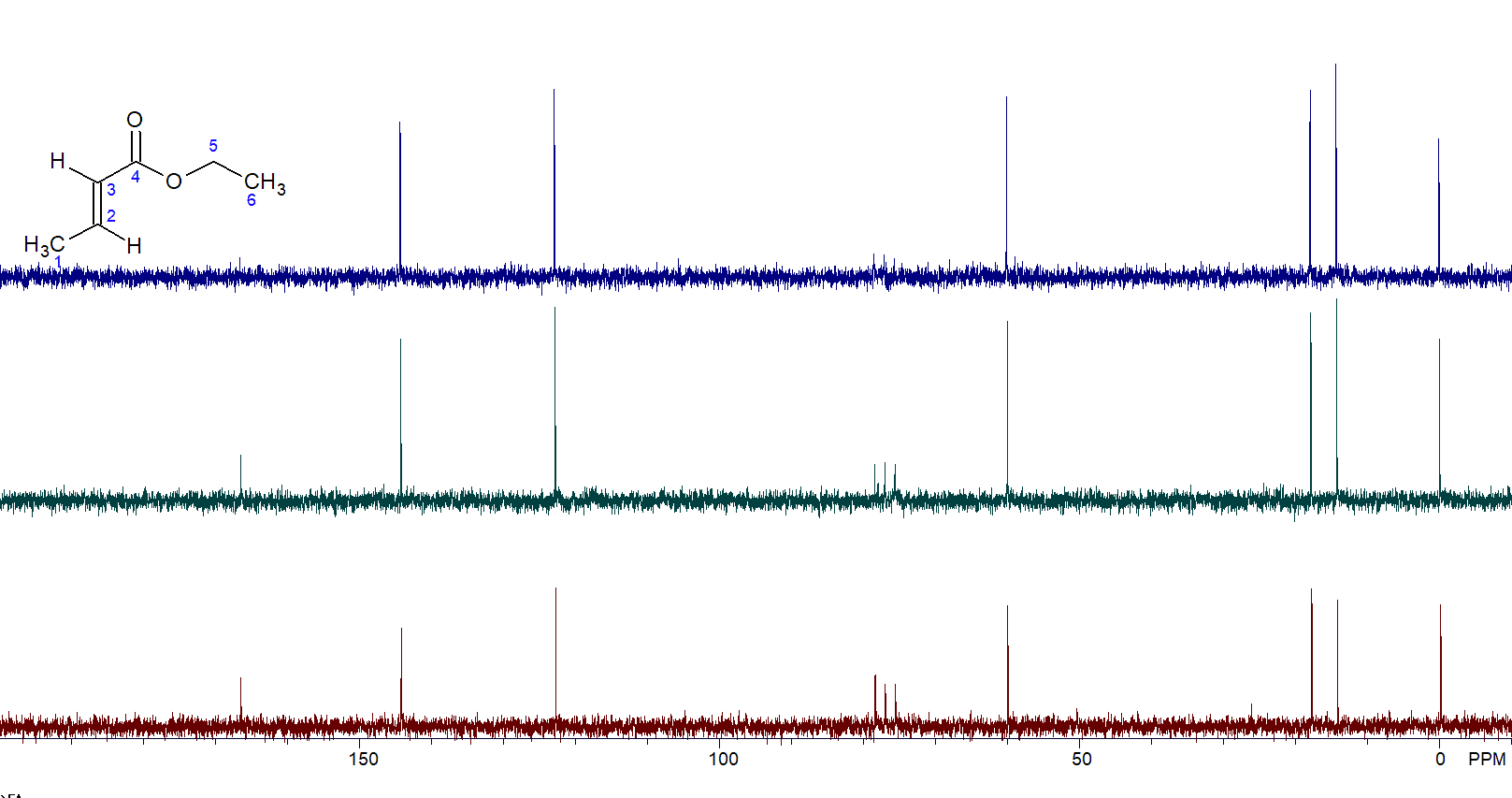
Figure 3. 90-degree pulse (top) vs. 60-degree pulse vs. 30-degree pulse (bottom).
Ensure an Adequate Relaxation Delay
After a pulse, the nuclei need time to get back to the state they were in before the pulse. Besides the acquisition time, which is normally a few seconds long, there is a relaxation delay (RD) that can be modified. A longer delay permits more complete relaxation and leads to a greater signal. However, increasing RD can lead to longer experiment times as well. Generally, the use of a RD that is 1-2 seconds longer than the standard RD will allow for greater relaxation of all the nuclei and increase the nuclear Overhauser effect (NOE). The NOE enhances the C13 signal intensity via polarization transfer from the decoupled protons.
Use the BAPR Program
Insignificant magnetic field drift that goes unnoticed in short experiments can cause problems during lengthy ones. That is true even for permanent aluminum nickel cobalt (AlNiCo) magnets, well known for their magnetic field stability.
Fortunately we have an elegant solution to mitigate field drift during very long acquisitions. The simple solution to this problem is BAPR. How does it work?
The BAPR program collects the desired number of scans in several blocks instead of a single acquisition. Meanwhile, during the time between blocks, the field can be shimmed and corrected for drift to keep the peaks in a relatively stable range. BAPR processing is quick, easy and can be done at any time during the experiment. Thus, it allows you to keep collecting data until the desired S/N is reached.
BAPR Results
Figure 4 shows the first several blocks of a BAPR program. The region shown is from about 127.5-133.5 ppm. The data is being analyzed while the program continues to acquire data. If the S/N is sufficient, you can stop the acquisition.
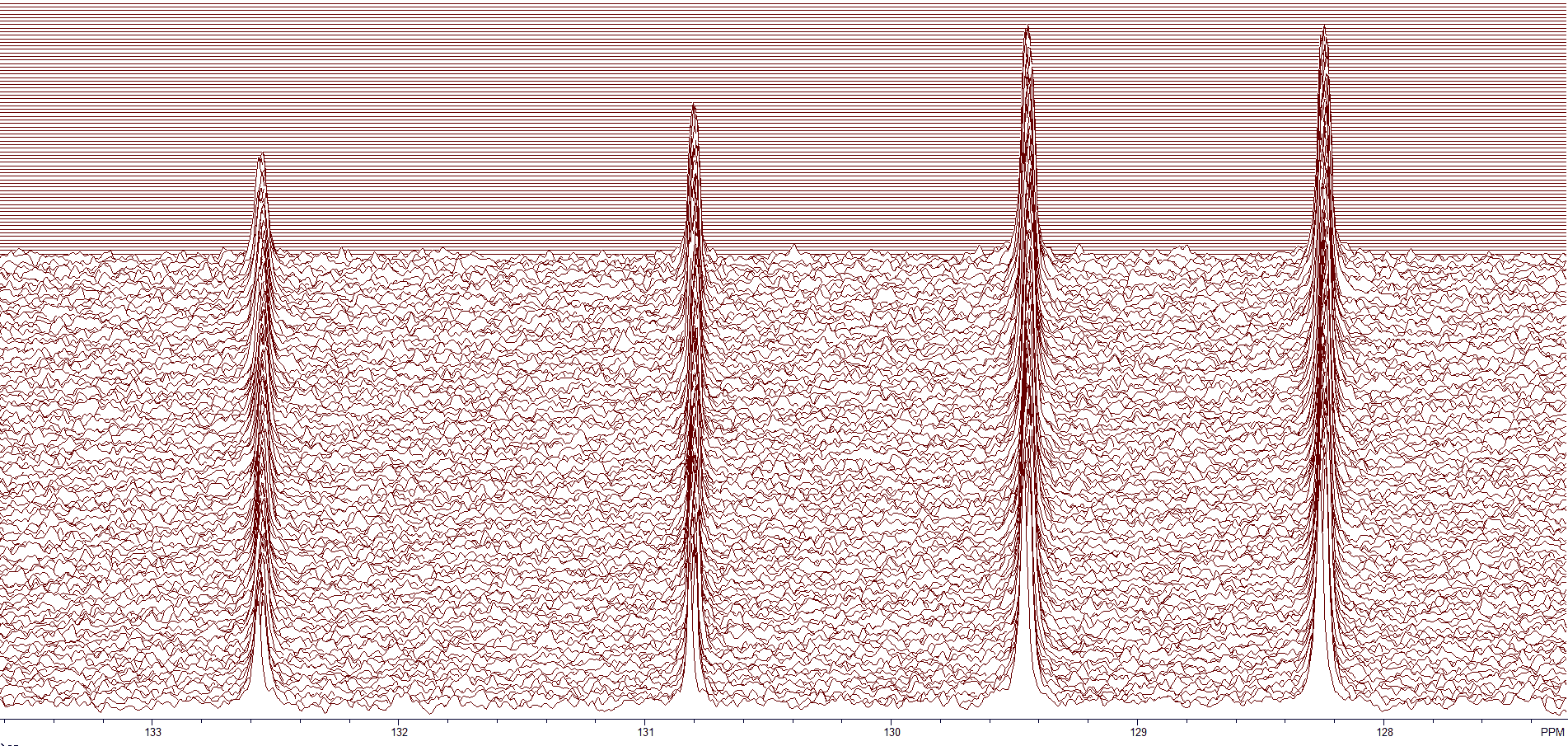
Figure 4. The aromatic region of a dilute sample.
Zooming in on the peak at 128.2 ppm (see Figure 5) shows the small variance in the chemical shift for the BAPR program. In general, the peaks are off by +/– 1.5 data points.
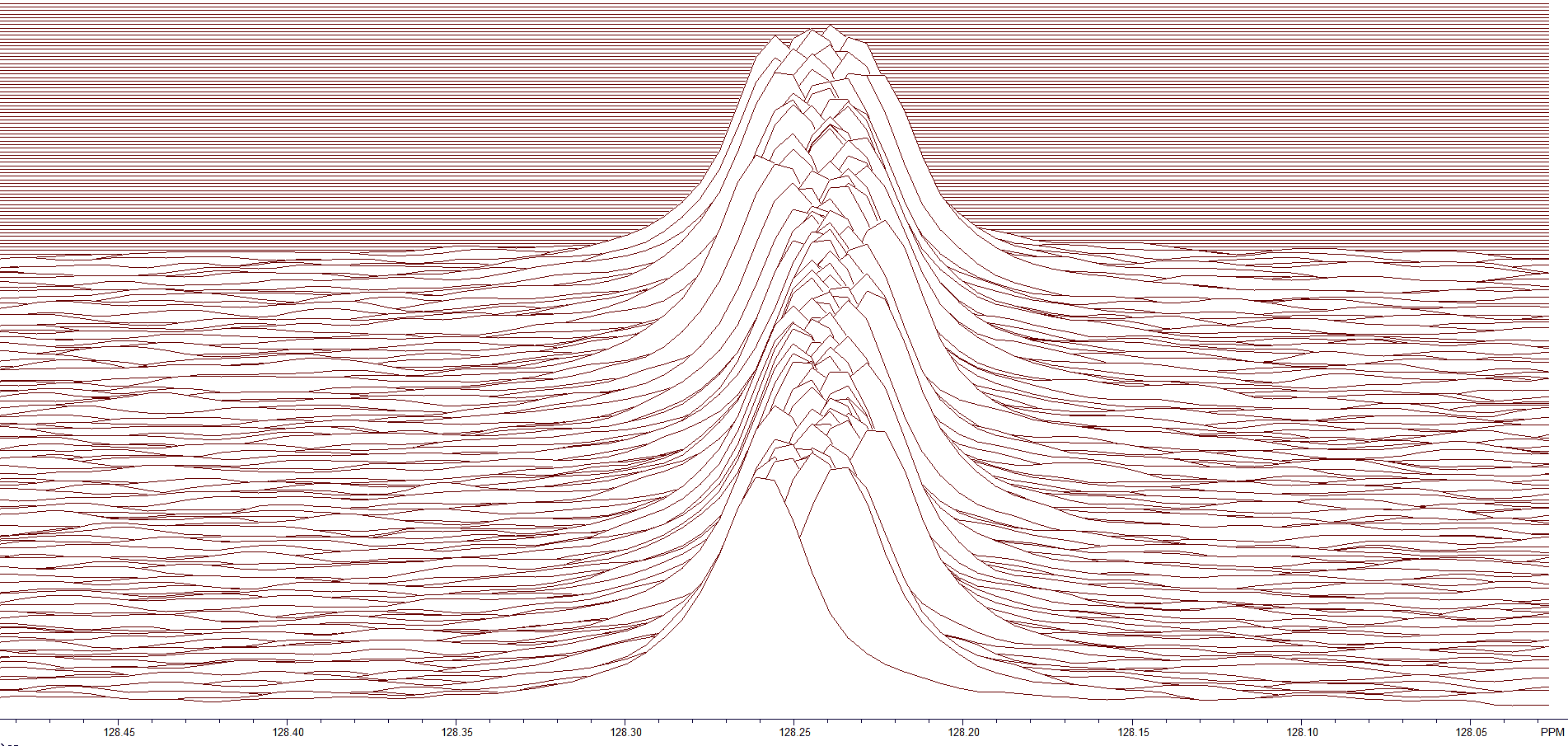
Figure 5. Zoom region at 128.2 ppm.
After peak registration, all the peaks align with the first slice of the BAPR (Figure 6). Notice that the variation in chemical shift is almost zero.
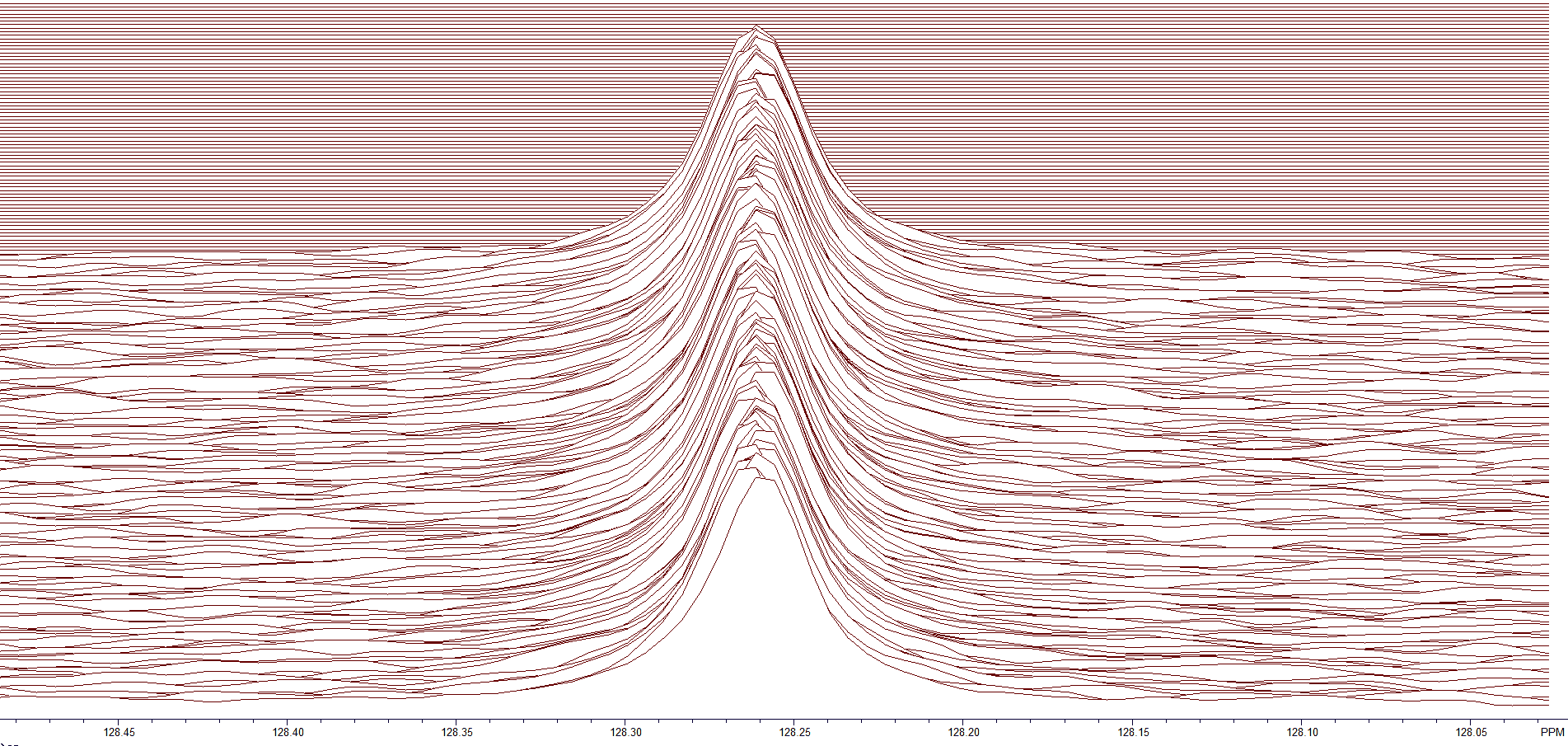
Figure 6. All peaks aligned using peak registration.
Once all the peaks are aligned (Figure 6), the peaks are summed for the final result. The overall S/N that is achieved is shown in Figure 7.
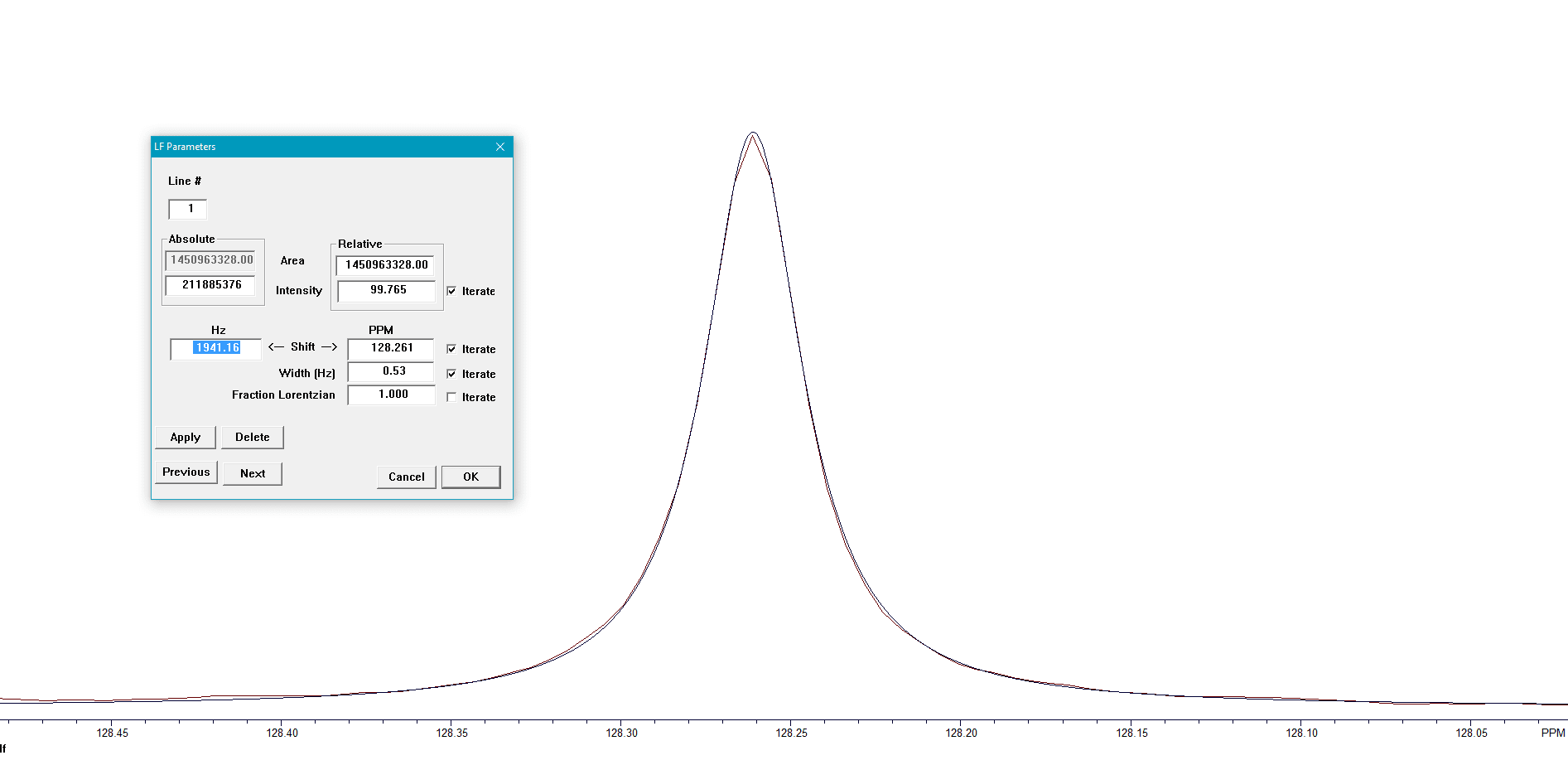
Figure 7. Results of the BAPR program.
